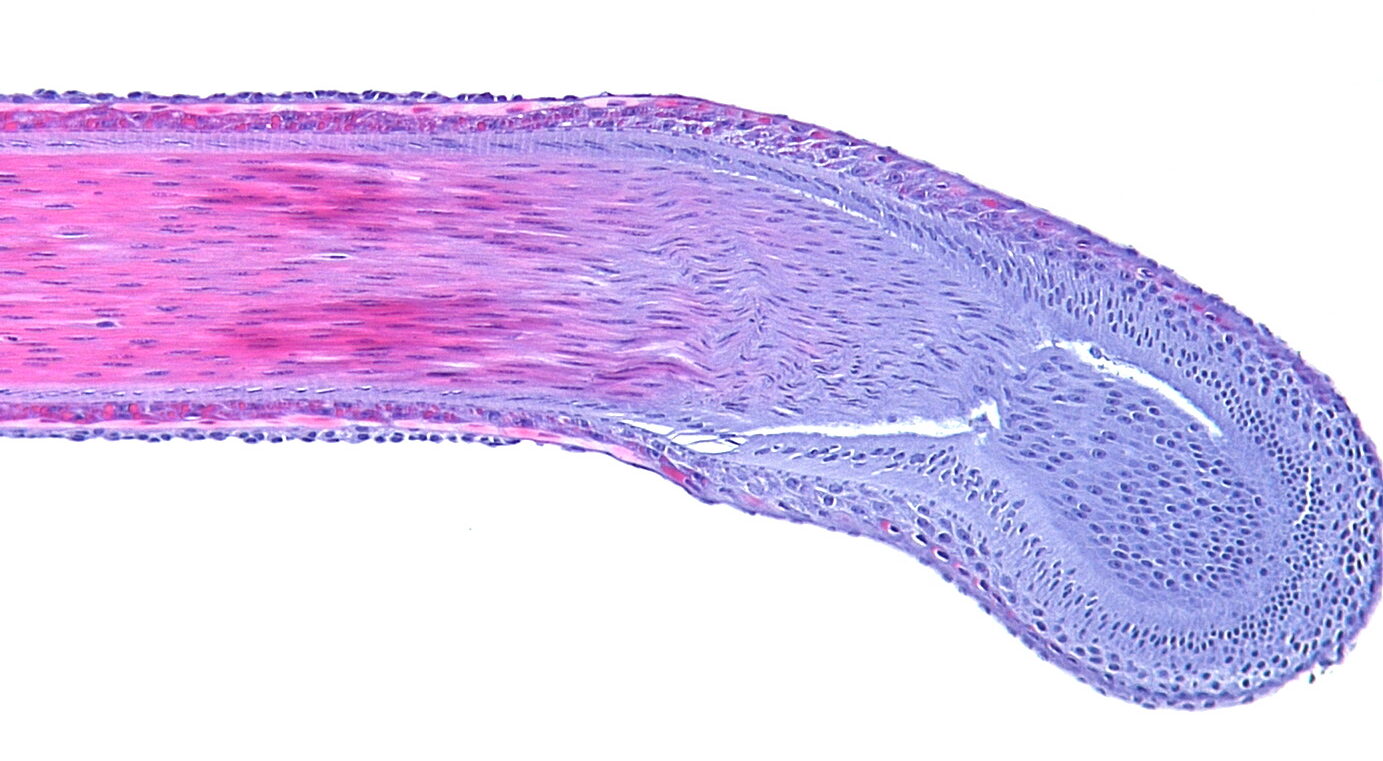
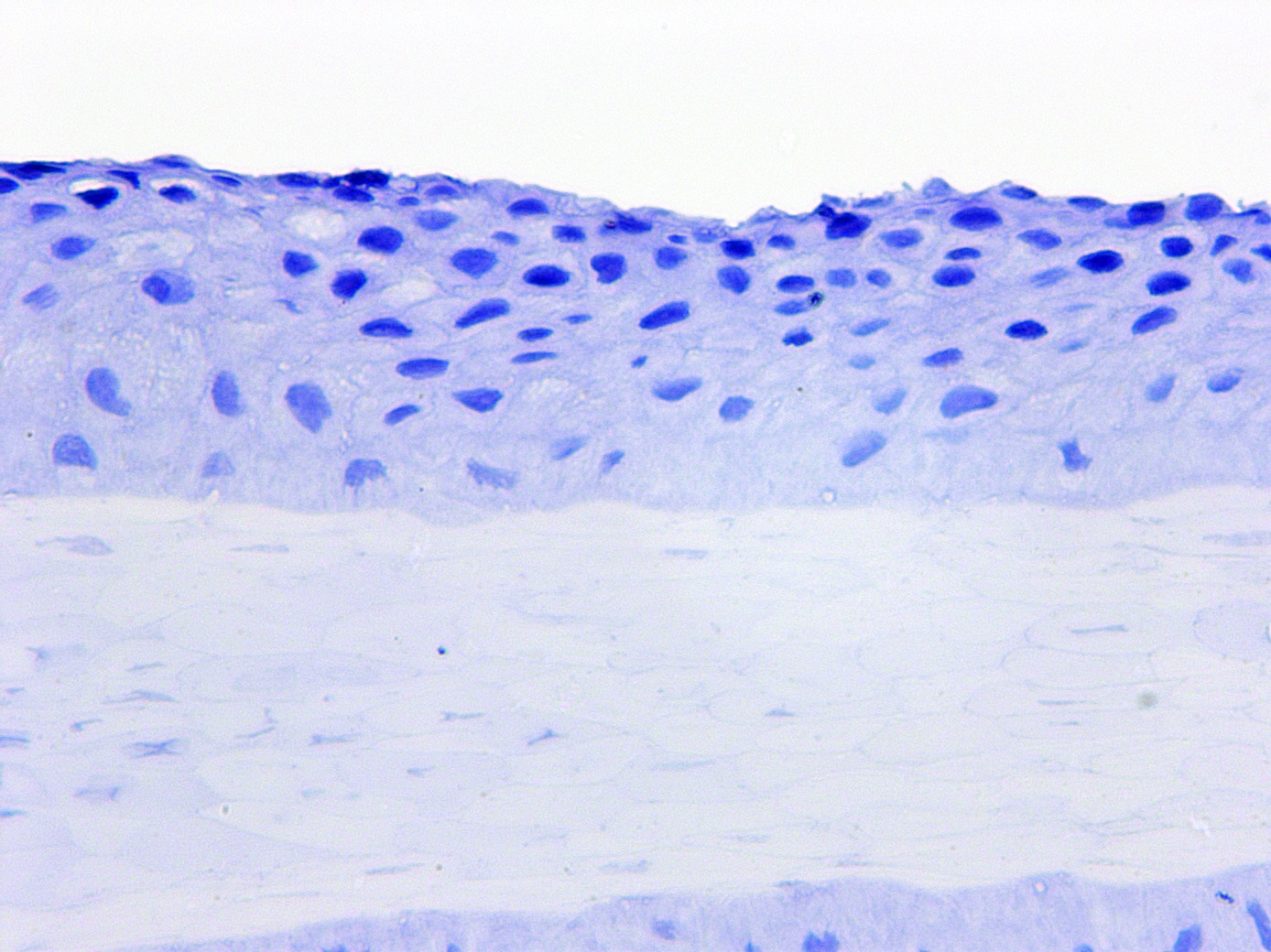
Hair is an ideal surrogate tissue for monitoring drug responses. It is easily accessible, plucking is simple, relatively pain free and yields multiple independent samples per timepoint. The scalp is the preferred source of plucked hair in humans, as most hairs in this region are in the anagen (active growth) phase of the hair cycle.
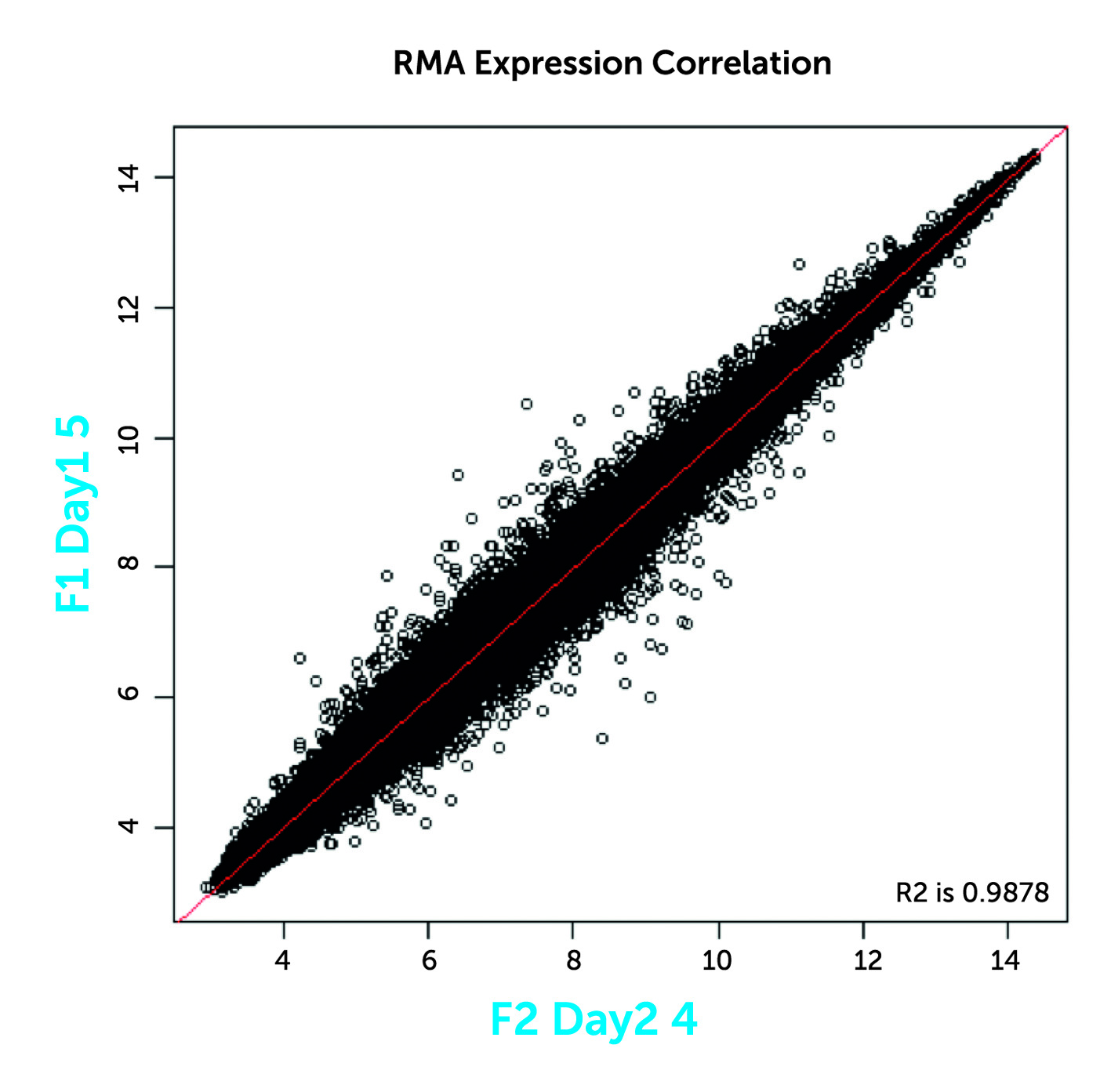
Proof of concept studies can easily be performed by placing plucked scalp hairs from healthy normal volunteers into media +/- the drug of interest for a given period of time. Hairs are then placed into a preservative solution and gene or protein expression changes evaluated. Direct changes to the target may be measured, such as phosphorylation, by immunohistochemistry, or changes induced in downstream gene pathways may generate a response signature.
The hair biomarker platform can assist drug development programs in monitoring pharmacodynamic response to treatment, duration of drug effect, dose response, confirmation of mechanism of action, patient stratification and hair growth.
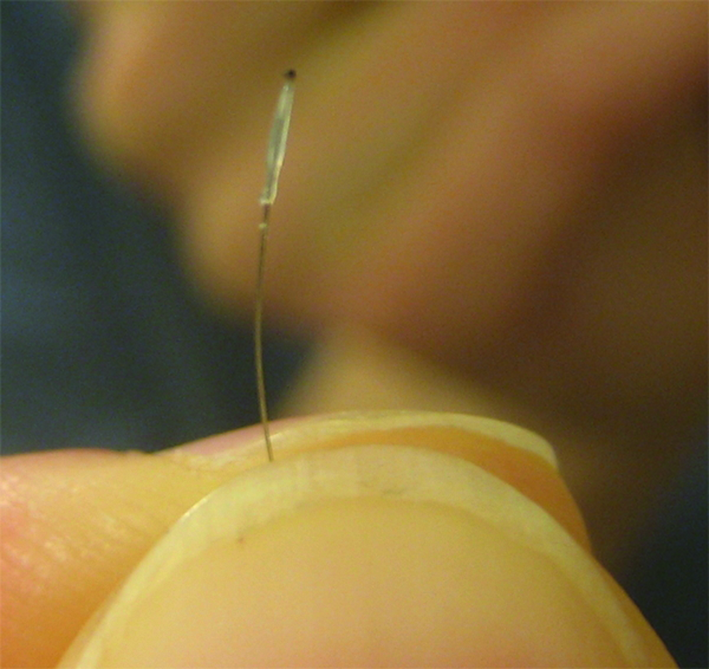
In the clinic, patient’s hair can easily be collected and placed into preservative solution and shipped to Epistem for similar analysis. Pre-clinical ex vivo studies can help determine intra- and inter-donor variability to ensure subsequent clinical studies are sufficiently powered. Confirmation of optimal storage duration for antigen stability can also be determined and used to inform the clinical trial protocol.
Epistem provides sampling kits for clinical studies, that include a collection guide to ensure that the samples are collected correctly and with optimal quality. These kits are designed to be easy to use, and instructions are provided to ensure that the hair is collected in a manner that is consistent with clinical trial requirements. In addition to providing sampling kits, Epistem offers remote training to ensure that hair is collected correctly. This training is designed to ensure that the samples are of the highest quality and meet the requirements of the study protocol.
All hair biomarker studies are performed in our GCLP-compliant histology and pharmacogenomics labs. Epistem has conducted GCLP accredited clinical studies in support of drug development pipelines for a number of major pharmaceutical customers.